Addressing the Main Issues
In this synthesis, Carson came across many problems. However, Carson followed his own advice. “Successful people don't have fewer problems. They have determined that nothing will stop them from going forward.” He worked with many reagents and struggled with questions of stereoselectivity. Below Carson has detailed the issues of synthesis he found most important.
What other reducing reagents could have been used?
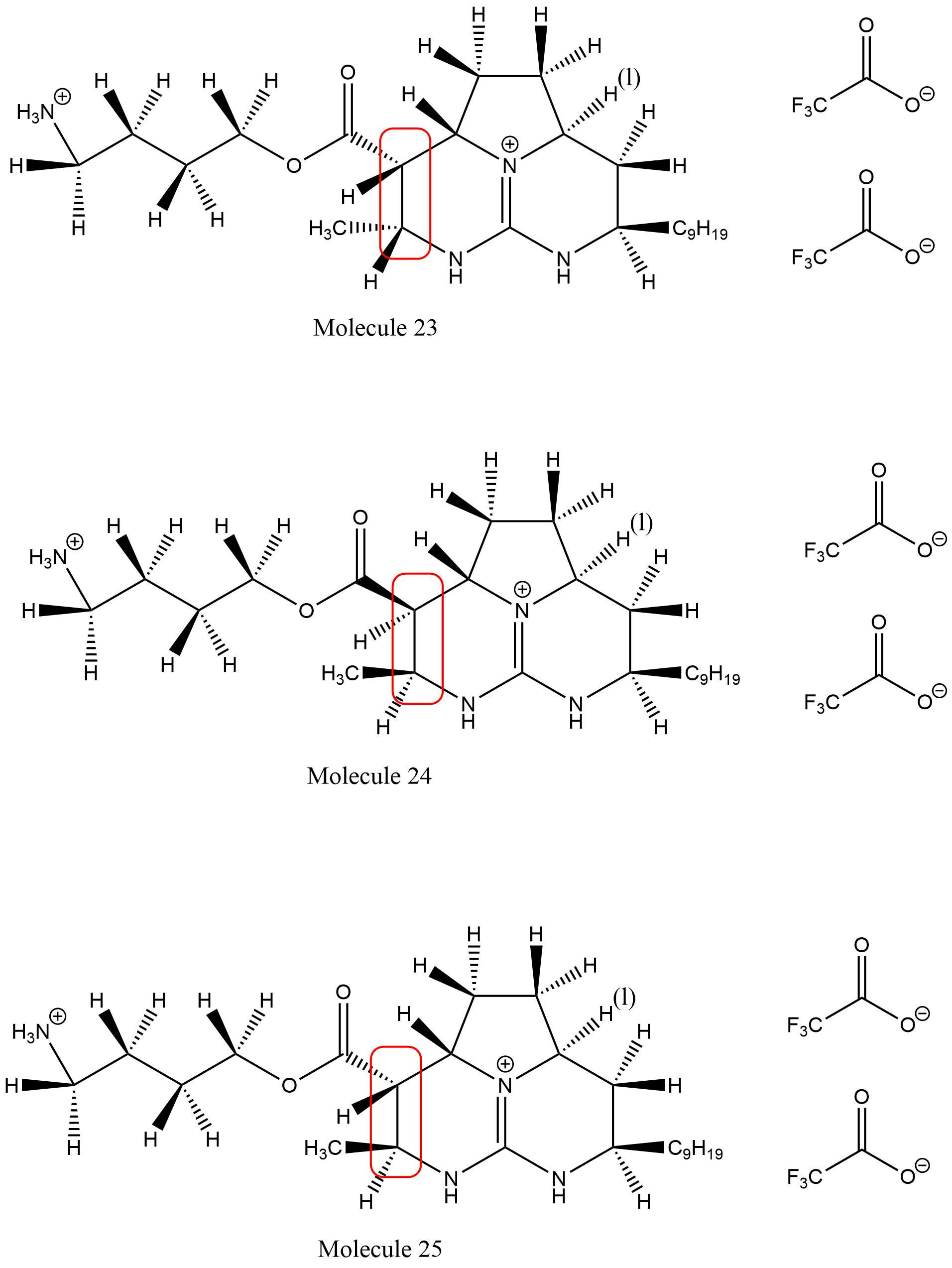
In the reaction, a rhodium on aluminum oxide (Rh/Al2O3) catalyst in methanol and water was used to reduce a carbon-carbon double bond. This reaction resulted in the syn addition of hydrogen atoms, with two stereoisomers forming in a 1:1 ratio. After some research, it was determined that the same result could be obtained from a variety of reagents. One of the sources cited by the main article, the Ly dissertation, showed that some other metal catalysts gave the same products but had slightly different stereoisomeric ratios. The other catalysts that could be used are Pd/C in hexanes, Rh/Al2O3 in DMF, and Rh/Al2O3 in hexanes. These reagents would give similar results but the ratios varied slightly. The ratios determined by the dissertation were 1:1, 5:1, and 10:1 respectively. These results indicate that although there were other possibilities for the reaction, the Rh/Al2O3 in methanol and water gave the best ratio of stereoisomers. These other reactions did not produce the correct ratio, yet still give the desired product.

Why the poor stereoselectivity?
In this experimental step, a hydrogenation of a double bond through the use of a rhodium on aluminum and oxygen catalyst occurs. This reaction gives a mixture of stereoisomers, which have methyl and ester substituents, both on a wedge or dash fashion. The inability for the reagents to be stereoselective is due to the structure of Molecule 18: it is relatively flat at the double bond. In a metal-catalyzed hydrogenation, the reagent approaches the catalyst metal in order to bond with the hydrogens. Because Molecule 18 is so flat at this double bond, it is equally likely to be reduced on the top or bottom side. Hydrogenation under these conditions will add predominantly syn, so mostly two stereoisomers are formed: predominantly both hydrogens are on a wedge (which is the desired product) or they are both on a dash (Molecule 24). However, there was a small amount of anti product formed giving molecule 25. This is why the reaction has such poor stereoselectivity.